Abstract
Background The antiphospholipid syndrome (APS) is characterized by the presence of antiphospholipid antibodies (aPL) and is associated with an increased risk of thrombosis and pregnancy morbidity. The Laboratory diagnosis of APS is difficult because most patients already use anticoagulants due to a thrombotic event, resulting in interference with the clotting assays used for lupus anticoagulant (LAC) testing. Nevertheless, the aPL profile defines patient management, making aPL testing warranted during anticoagulant therapy. Sometimes, cessation of anticoagulant treatment is necessary to perform the lupus anticoagulant (LAC) test, which is unfavorable.
The thrombin generation (TG) test is a functional and global hemostatic test. Clinical studies have shown that increased TG is associated with thrombosis, also in thrombotic APS patients. We have previously shown that the artificial intelligence method neural networks can be used to diagnose untreated APS patients from a group of healthy, auto-immune disease, and thrombosis controls with high accuracy. At the moment of aPL testing, many patients are already on anticoagulants, hampering the detection of LAC. Therefore, we developed a neural net (NN) for diagnoses APS in a cohort of anticoagulated subjects, based on the TG test.
Methods Antiphospholipid antibodies positivity was diagnosed according to the Sapporo-Sydney guidelines. TG was measured using the calibrated automated thrombogram assay (CAT) on platelet poor plasma with PPP reagent (intermediate concentration of tissue factor) in 48 APS patients and 64 control non-APS patients, both treated with vitamin K antagonists (VKAs) TG was explored in more depth by quantifying parameters of prothrombin conversion and thrombin inactivation, using thrombin dynamics (TD) analysis, and assessing the sensitivity of the activated protein C pathway through the addition of thrombomodulin (TM).
Five NNs were developed and the most accurate NN was selected for clinical validation based on its sensitivity and specificity. Input parameters for the NN were TG parameters lag time, peak, endogenous thrombin potential, time-to-peak, and velocity index, and TD parameters total prothrombin conversion, maximum prothrombin conversion rate, thrombin-antithrombin complex formation, and thrombin-α2-macroglobulin complex formation, and the inhibition of TG and TD by TM. The top performing NN was clinically validated using the TG and TD data acquired in the validation cohort, consisting of 33 APS patients and 62 controls, both on VKA therapy, 49 auto-immune disease controls, 38 thrombosis controls, 92 patients visiting the hospital for other indications and 37 healthy subjects. All control subjects were negative for aPL.
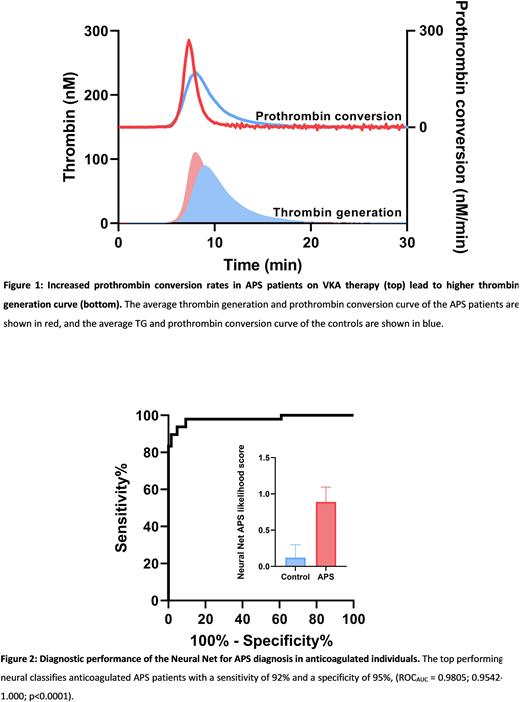
Results TG was slightly increased in APS patients compared to controls. VKA therapy abolished the typical lag time prolongation seen in non-anticoagulated APS patients due to the LAC effect. The rate of prothrombin conversion was significantly increased in anticoagulated APS patients compared to controls, indicating a more prothrombotic phenotype.
The selected NN classified APS patients with a sensitivity of 92% and a specificity of 95% in the developmental cohort consisting of APS patients and controls on VKA antagonists (ROCAUC = 0.9805; 0.9542-1.000; p<0.0001). On average, controls and APS patients were assigned an APS likelihood score of 12% and 89%, respectively.
The NN was clinically validated in a separate validation cohort of 311 individuals, including 33 anticoagulated APS patients, 62 anticoagulated controls, 38 thrombosis patients, 49 auto-immune disease patients, 92 hospital controls, and 37 healthy controls. The sensitivity of the NN for the detection of APS was 85%. The overall specificity of the NN was 93%, and 100%, 96%, 96%, 92% and 76% respectively in healthy controls, auto-immune disease controls, hospital controls, anticoagulated controls, and thrombosis controls.
Conclusions We developed a NN that accurately classifies APS under anticoagulant treatment. Therefore, this NN could be an alternative for diagnosing APS patients already treated with VKAs.
Disclosures
de Laat-Kremers:Synapse Research Institute: Current Employment. Ninivaggi:Synapse Research Institute: Current Employment. de Groot:Synapse Research Institute: Consultancy. de Laat:Synapse Research Institute: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal